Data Journalism Data Journalism Top 10
Data Journalism Top 10: How COVID-19 Changed the US, Race for the North Pole, and Visualizing Coronation in the UK
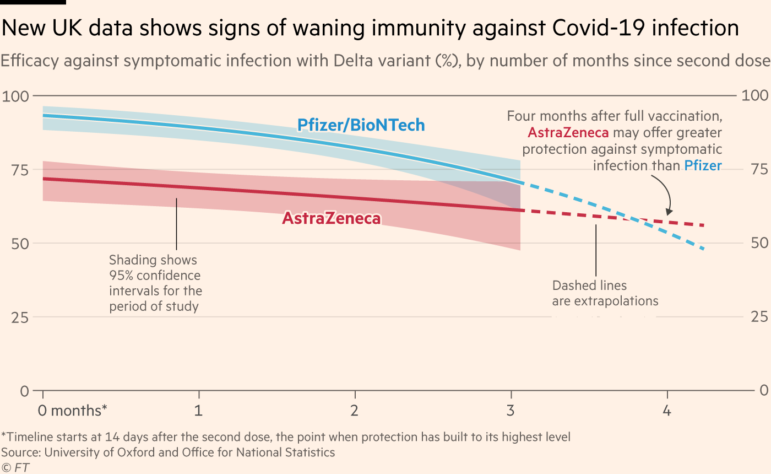
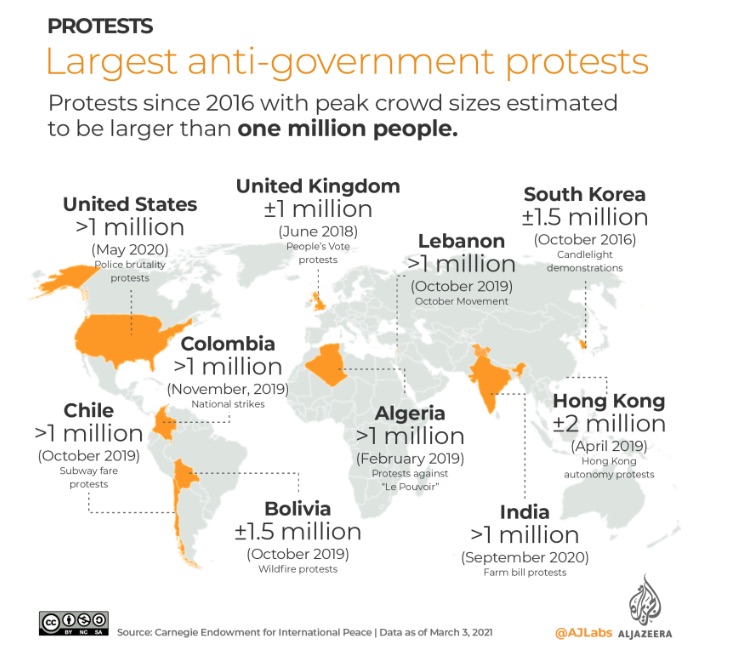
This week GIJN’s Top 10 in Data Journalism features the Wall Street Journal’s dive into how COVID-19 changed the daily lives of Americans, the Straits Times on Singapore’s gold medal history at the Southeast Asian Games, and how data journalists visualized the coronation of King Charles III.