

Document of the Day: A Look at Pfizer’s Vaccine Press Release
Read this article in
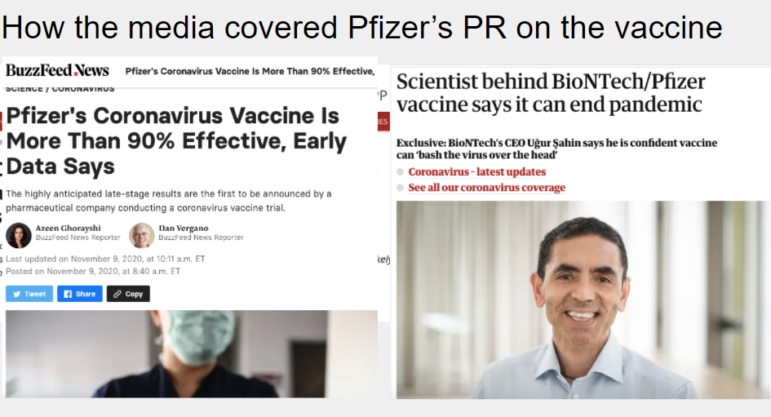
A November 18 press release, claiming a safe vaccine that protects 95% of people against COVID-19, by pharmaceutical companies Pfizer and BioNTech was greeted with excited headlines.
 In fact, this was a rare case in which — in the words of health investigative reporter Serena Tinari — a corporate media release represented “the biggest news of the week, worldwide.”
In fact, this was a rare case in which — in the words of health investigative reporter Serena Tinari — a corporate media release represented “the biggest news of the week, worldwide.”
November saw a flurry of similar announcements related to COVID-19 vaccines. There was a separate release published November 9 from the same US-German corporate collaboration, another on November 16 with similarly promising claims by biotech startup Moderna, and one on November 23 by AstraZeneca, as global deaths in the pandemic reached about 1.4 million.
What should reporters look for in such releases? We asked Tinari and Catherine Riva, co-founders of the nonprofit health watchdog Re-Check.ch and authors of the just-released GIJN guide, Investigating Health & Medicine. They warn that press releases are no substitute for published peer-reviewed studies. And, discussing the Pfizer and BioNTech release in a GIJN webinar, they balanced cautious optimism with the provision that the release relied on interim results from a trial in an accelerated setting.
“We are all really looking forward to having a great vaccine and drug here, but we are also very cautious, and the press release unfortunately is not enough to draw any conclusions on the safety and efficacy of the vaccine,” said Riva. “The full trial data has yet to be peer-reviewed and published.”
Riva and Tinari compared the release to the trial protocols and raised these questions:
- The release states that vaccine efficacy in people over 65 years of age was over 94% — an achievement that garnered headlines around the world. (“Efficacy” refers to the performance of an intervention under controlled study conditions.) But the efficacy data in the release is based on only 170 of the 43,000 participants in the trial. How many of the 170 were over 65 years old? Says Riva: “We will have to wait for the publication, because nothing in the press release allows us to answer this question.”
- The release says that 41% of global participants, and 45% of participants in the United States, are between 56 and 85 years of age. But what is the proportion of these participants in the 170 study participants included in the press release? “If there are only a few cases, it would be quite premature to draw conclusions about the vaccine’s efficacy for all the 43,000 participants,” notes Riva.
- The release distinguishes between “confirmed” and “severe” cases. But what should count as a severe case? In the study protocol, investigators defined “confirmed” to mean a positive SARS-CoV-2 test plus at least one symptom, such as fever or cough. They defined “severe” to mean a positive SARS-CoV-2 test plus a life-threatening factor, such as admission to an Intensive Care Unit, or ICU. “But what is considered a ‘COVID case’ can vary considerably depending on the country or region,” says Riva.
- The press release states: “Data demonstrate vaccine was well tolerated across all populations with over 43,000 participants enrolled; no serious safety concerns observed…” Riva points out that safety and side effects were not assessed for the 43,000 participants, but rather only for a subset of 8,000 adult participants.
- While the “fast track” approval process was established with the noble purpose of rapidly delivering needed treatments, reporters need to know if procedures were shortened or skipped.
Screenshot: Re-Check.ch’s Senera Tinari says vaccine development stories based on press releases and interim results should seek to avoid any impression that the facts were sourced from published, peer-reviewed studies.
“Vaccines have to be especially safe, because normally they are given to healthy individuals,” says Tinari. “And we need to learn from the past. Remember, ten years ago, for H1N1 swine flu, GlaxoSmithKline’s vaccine Pandemrix was supposed to be safe and effective, but it turned out to have harmful effects on kids.”
Riva adds that Pfizer and BioNTech’s disclaimers in the press release note that these are interim results. “The study’s conclusions could change,” she stresses. “The numbers communicated by these two pharmaceutical companies seem encouraging, no doubt, but every claim should be considered very carefully because of the numerous limitations.”
Additional Reading
Webinar Series: Investigating the Pandemic
A GIJN Guide: Investigating Health and Medicine
 Rowan Philp is a reporter for GIJN. Rowan was formerly chief reporter for South Africa’s Sunday Times. As a foreign correspondent, he has reported on news, politics, corruption, and conflict from more than two dozen countries around the world.
Rowan Philp is a reporter for GIJN. Rowan was formerly chief reporter for South Africa’s Sunday Times. As a foreign correspondent, he has reported on news, politics, corruption, and conflict from more than two dozen countries around the world.









