
How Publishing Investigations as Books Can Add Depth, Increase Visibility, and Fight Censorship
In this story, several investigative journalists from Mexico speak about the experience of turning their exposés into books.

In this story, several investigative journalists from Mexico speak about the experience of turning their exposés into books.

Featuring titles that address historic wrongdoing, unpack corporate secrets, and reveal misconduct that the powerful would rather remain hidden.
The year 2022 has seen a number of noteworthy books about investigative journalism, from going undercover to dig into corruption to investigating the underbelly of the global migration crisis, and from a deep dive into dodgy COVID contracts to a book from Nobel Peace Prize winner Maria Ressa.
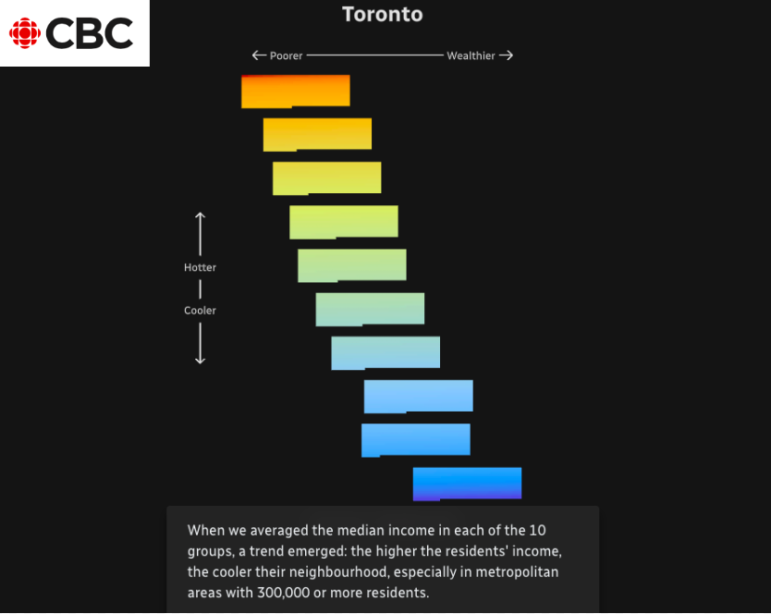
As parts of the world endure record-breaking temperatures, a highlight from the world of data journalism this week involves an analysis of how “heat islands” in Canadian cities vary based on economic strata. Our weekly Top Ten in Data Journalism also looks at the global spread of Pegasus spyware, digital inequity in the US, and how the COVID-19 pandemic affects school children in Latin America.

For this week’s Friday 5, where GIJN rounds up key reads around the world, we found stories about freelancers commissioned to write for a massive Russian-backed disinformation campaign, how to (not) get your pitch read by an editor, and a guide for reporting on US elections.
The dominance of the English language might be skewing local and international reporting — as well as the global media development space — writes GIJN’s Managing Editor Tanya Pampalone. She wrote the opening essay for Hostwriter’s new book “Unbias The News: Why Diversity Matters for Journalism.”