
Health and Medicine Guide: Chapter 4
Read this article in
Guide Resource
Investigating Health and Medicine
Chapter Guide Resource
Health and Medicine Guide: Preface
Chapter Guide Resource
Health and Medicine Guide: Introduction
Chapter Guide Resource
Health and Medicine Guide: Chapter 1
Chapter Guide Resource
Health and Medicine Guide: Chapter 2
Chapter Guide Resource
Health and Medicine Guide: Chapter 3
Chapter Guide Resource
Health and Medicine Guide: Chapter 4
Chapter Guide Resource
Health and Medicine Guide: Chapter 5
Chapter Guide Resource
Health and Medicine Guide: Appendix
First, Do No Harm. Reporting About Safety
Once a pharmaceutical product — a drug, vaccine, or medical device — has gone through the different testing phases, and the approval process with regulatory agencies, it hits the market and can be prescribed and sold. Serious adverse events can appear when the product is widely used for the first time by real patients. This relates not only to potential flaws in scientific research and problems related to regulatory approval and reporting in scientific journals. It is also sometimes a matter of numbers: If you test safety on 5,000 patients, an adverse event arising in one of 20,000 will become apparent when many more patients use the product. Therefore, the first 10 years after a drug is approved are considered especially important to spot harms.
Tip 1: Assess the Evidence
Editors love stories about the damage caused by drugs, vaccines, and medical devices because they are popular with audiences. There are a plethora of potentially good stories, but many pitfalls await and careful reporting is required.
First, one needs a systematic understanding of drug development and testing, including a review of the related literature and data. As we have seen in the first chapter, a few thousand people or so will have tested a medical product in a controlled environment before it goes to market. That’s why, although there are exceptions, “new drugs” are generally considered less safe than older ones; they just aren’t as well understood medically.
What is defined as “post-marketing surveillance” by regulatory agencies is important. This includes pharmacovigilance, a type of monitoring which the WHO defines as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem.”
When pharmacovigilance is based on voluntary reporting, this can be a major issue. There are several ways for doctors, nurses, pharmacists, and patients to report suspected side effects, including by reporting them directly to regulators. If such reports are considered credible, national regulatory bodies may forward them to the WHO Programme for International Drug Monitoring in Uppsala, Sweden. The reports are not public; as a journalist you can have access to them if a patient or a doctor shares them with you. Regulators may provide statistics from the reports, but even though personal identities are masked, the full reports are still considered highly confidential.
Underreporting of the adverse reactions to medical products is perhaps the most troubling weakness of this system. Only a tiny fraction of adverse events are reported, according to a comprehensive study from 2006.
Beware of relying on a single report of an adverse reaction. These are called “signals” that tell experts to do further research into causality. Many other factors may have contributed. A single report needs to be evaluated along with other evidence.
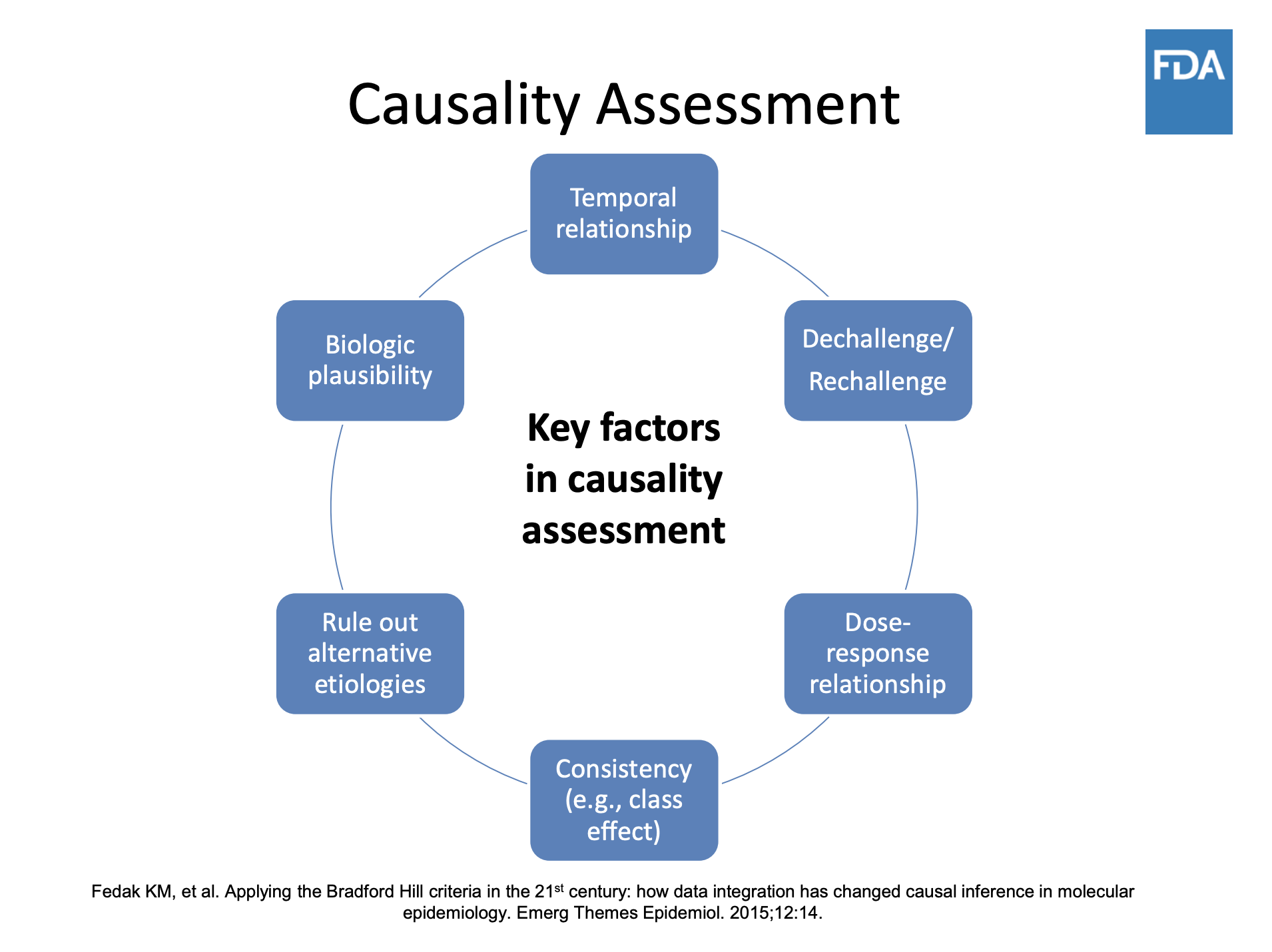
Illustration: US Food and Drug Administration
Typically, news stories affect the reporting of adverse event reporting (called AER). If there is media reporting of a drug’s alleged dangers, in the weeks that follow regulatory agencies will record a significant increase in reports. Events reported by patients, although considered of lower quality than those filed by medical professionals, have real potential to improve the system, as pointed out in the article Patient Reporting Is the Future of Pharmacovigilance by Sten Olsson, then-president of the International Society of Pharmacovigilance. Keep in mind that exposing evidence will not necessarily lead to a drug being pulled off the market. Regulatory procedures are often more complicated. Your reporting may push regulators to modify the drug label, a process where extensive negotiations with the marketing company will take place.
Tip 2: Retrieve Data and Talk to the Victims
There is a big difference between the safety data collected during development and approval and the data that emerges post-marketing.
All reports on the side effects of a drug from a WHO member country from 1968 onwards are stored in VigiBase and remain available, even if the products are no longer on the market. You can request access to the data available by filing a request with national regulatory agencies.
The data come with many caveats and are truly hard to analyze, as a single case can be recorded under several different codes. However, health stories need real humans speaking about the harms or damages they have experienced. “Case reports” are always anonymized, so when you receive data from pharmacovigilance officers, you need strategies to find such alleged victims. One is through doctors: They may have a patient who is willing to talk to journalists. Or they may know another doctor whose patient experienced that side effect.
Social media sites offer many possibilities via crowdsourcing, as do websites and newsletters of your media outlet. Posting an open call that states clearly that you are investigating the potential harm, or patients’ experience with a pharmaceutical product, will expose your ongoing project but will give you the greatest chance of getting in touch with potential protagonists.
So, let’s imagine you came across a potential harmful product and even identified potential characters for your story, patients that allegedly were harmed by that product. What do you do next? The key is to independently assess the evidence and compare your findings with studies published in the literature.
Often victims’ stories have not been reported at all. Telling their stories may provoke responses from patients or families contacting your newsroom. Collect all the cases, complete with medical details, and don’t forget to ask them to sign a waiver of their physician-patient privileges. Without this waiver, your investigation will be delayed, as doctors and hospitals will refuse to respond to your requests until you have the waiver. Be aware that national laws protect the privacy of patient medical information, and some are quite strict.
When you have a complete picture of specific cases, present the evidence to the regulators. Experience has shown many times that it’s useful to contact them before your investigation is concluded and well before publication. If your methods are solid, and you have discovered facts that are in the public interest, it’s actually in the regulators’ best interest to support your work as far they can. Of course, they won’t give you the names of patients or information that puts them in a bad light. But they may point to aspects you might have underestimated (say a study, or data, or a specific rule applying to the case), so they can help you avoid errors in your reporting. Sometimes, they may even hint at the pharmaceutical company or the doctors’ organization that played a role in delaying or hindering regulators’ work. At the same time, the industry often uses a strategy of information overload when responding to queries from investigative journalists. Expect lengthy statements and references to studies replete with scientific jargon.
Our To Die for the Skin investigation for the Swiss public broadcaster on acne drug Accutane’s psychiatric side effects, led to a wave of patients and their families sending their testimonies. Out of over 300 reports we received, 61 cases were included by the Swiss regulatory agency Swissmedic in the national and global database on drug safety. The agency also issued an update to prescribers and patients on the drug’s psychiatric risks.
Similarly, the medical devices investigation by Jet Schouten, a journalist with Dutch public broadcaster AVROTROS which inspired the ICIJ Implant Files project was sparked by the many testimonies the broadcaster received after publishing its first story.
Finding sources of all kinds for such an investigation can be complicated. Crowdsourcing and social media searches are key. Use advanced research tools like those listed in the GIJN guide.
Court proceedings can be a trove of information, as well as a place to look for sources, particularly if you are investigating harmful effects and damage.
Getting too close to your sources is never a good idea; it certainly should be avoided when reporting about drug safety. When you are presented with injustice, patients who weren’t properly informed, regulators who knew but didn’t act, potential or real conflicts of interest, and highly knowledgeable experts with huge reputations, it is tempting to rely too much on one or two sources. Your duty is to assess the evidence and expose wrongdoings, not make new friends.
It’s also important to use careful, accurate language, and not to hype your drug safety story. Inflammatory language simplifying the risk-benefit ratio and distorting the scientific evidence will leave space for the authorities to ignore the matter. Poor quality reporting could even inhibit intervention by regulatory authorities. Patients may stop taking a drug without talking to their doctor; this could be dangerous to a their health. Hyped stories may lead to actions which are not evidence-based, such as pulling a drug from the market instead of modifying prescription guidelines to make sure it is taken correctly.
Tip 3: Expose Fraud, Scientific Misconduct, and Medical Malpractice
From nursing homes to research labs, from public hospitals to medical practices, health care is affected by fraud and misconduct. Many of the cases would not have come to light without the wrongdoing being exposed by victims, prosecutors, human rights advocates, and journalists. Some of this work is inspirational and instructive.
In some cases, it was necessary to go undercover. In 1887, American journalist Nellie Bly pretended to be mentally ill to investigate reports of brutality and neglect at a New York psychiatric institution. Fast forward over 100 years, and Naziha Syed Ali also went undercover in Pakistan to dig deeper into organ trafficking for Doctors, Police and Middlemen. So, too, did a BBC Africa Eye team led by Solomon Serwanjja for Stealing from the Sick, an investigation exposing the black market of pharmaceuticals in Uganda.
Victims’ testimonies are key, as in Deborah Cohen’s BBC story on stem cell experiments, or in the Thomson Reuters Foundation investigation led by Roli Srivastava Missing Wombs: The Health Scandal Enslaving Families in Rural India.
Medical malpractice can be analyzed by combining data with victims’ testimonies, as Peruvian outlet Ojo Público is pursuing with the project Cuidados Intensivos. Or see journalist and former molecular cell biologist Leonid Schneider’s investigation into an alleged case of scientific misconduct involving trachea transplants. The story is also featured in Benita Alexander’s documentary “He Lied About Everything” (Discovery) and Bosse Lindquist’s three-part documentary “The Experiments” (SVT). Another good read is Tide of Lies by Kai Kupferschmidt about the long journey of researcher Alison Avenell in exposing a major case of scientific fraud that highlights the many problems affecting biomedical journals.
One disturbing example can be found in Sushma Subramanian’s in-depth piece for Slate, Worse Than Tuskegee, on how in the 1940s American researchers infected Guatemalans with syphilis and gonorrhea, then left without treating them. The case emerged in 2003 thanks to Susan Reverby, a historian at Wellesley College. The Wall Street Journal’s John Carreyrou, who won a 2015 Pulitzer Prize for his Medicare Unmasked series, began a groundbreaking investigation that same year on blood testing firm Theranos that resulted in a new series, indictments, and his book “Bad Blood: Secrets and Lies in a Silicon Valley Startup.”
There is much to investigate in the area of so-called “corporate crime.” Two references guaranteeing you sleepless nights: Peter C. Gøtzsche’s book “Deadly Medicines and Organised Crime” (extract on BMJ: Big Pharma Often Commits Corporate Crime, and this Must be Stopped) and Public Citizen’s Twenty-Seven Years of Pharmaceutical Industry Criminal and Civil Penalties: 1991 through 2017.